Device Quality Inspection Systems
100% automated visual inspection for medical device and pharmaceuticals. Increase yield, protect safety and boost profitability.
Fully automated visual quality control of medical device and pharmaceuticals production.
Patient safety starts with the production of primary medical devices. Guarantee quality control and check every medical device component at speed with IVS automated visual inspection.
State-of-the-art inspection technology at process speeds, allowing you to achieve outstanding production control – assuring 100% patient safety and early detection of manufacturing faults in your process.

Why you need our Device Quality Inspection Systems & Machines
Discover the benefits of IVS providing the automated visual inspection of your products
GMP-compliance
Our integrated audit trails, user management and automated inspection fully comply with the requirements for GAMP validation – as well as requirements of CFR 21 Part 11. We provide the full validation paperwork with our systems.
State-of-the-art Vision
Our machines utilise artificial intelligence deep learning technology, providing the highest quality control decisions automatically. Never miss another failure with the very latest in computer vision automation and control.
Industry Knowledge
We’ve been installing automated vision inspection and vision systems into medical device and pharmaceutical production lines for 20 years, so we understand your industry. Our expert engineers can advise the best solution for your production.
Features of our Device Quality Inspection Systems
Explore some of the key features and benefits of our device quality inspection machines for automated quality control
Comprehensive Defect Detection
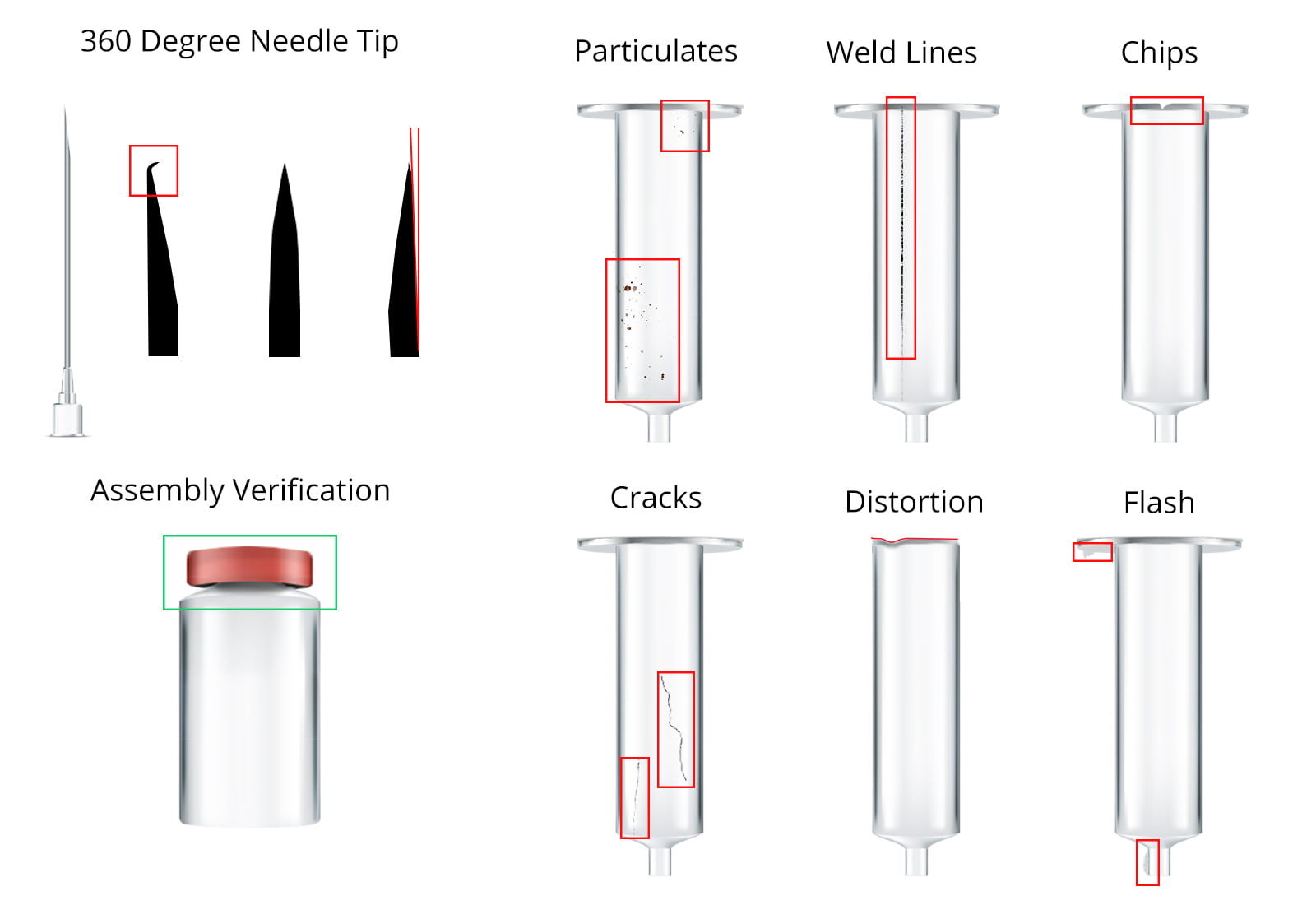
IVS provide a comprehensive range of automated visual inspection systems and machines for medical device and pharmaceutical products. Automatically detect black inclusions, scratches, deformation, warpage, flash problems, short detection, cracks, bubbles, caps, crimps, fill levels, needle sharpness, cosmetic defects and measurement issues on all devices.
Feel protected with our 100% quality checks. Verify assemblies, check needle angles, detect mould distortion, identify particulates. Comprehensive, validated automatic inspection providing high speed analysis and fault detection at your production speeds.
Versatile Visual Inspection Equipment
Tailored, user-friendly solutions for the inspection of syringe bodies, medical tubes, medical caps, plungers, barrels, stoppers and crimps – from vials, cartridges, medical device bodies and needle tips through to injection systems, inhalers parts, specialist medical devices, assemblies and caps.
All areas of medical device and pharmaceutical production are covered, from the automated checking of injection moulds directly out of the tool, through to in-line quality checks of assembly processes, final inspection and packaging. Whatever stage of the production process you are at, we can help with accurate and reproducible inspection results.


Modular Machines
Our user-friendly vision inspection systems & machines are easy to operate and maintain. Ultra-high resolution state-of-the-art imagery allows 100% continuous automated visual inspection. Tailored to your product requirements our modular design and construction fit into your production environment.
Stand-alone fully automated high speed vision inspection machines with integrated hopper, bowl or robot feeds, with precise dial plates for precision quality control checks and sorting of devices, components and pharmaceutical products – fully validated to GAMP standards.
Production Control
Use IVS inspection solutions to maintain control of processes and yields. Insightful dashboards display at-a-glance process overviews, providing real-time data of the most pressing quality issues impacting yield. Comprehensive reporting delivers feedback on reject issues, spikes in quality concerns and corrective actions needed.
Designed with maintenance in mind, providing both process information and maintenance feedback. Your engineers will save time with real-time monitoring and analytics from the shopfloor. Stop quality escapes earlier on the line and achieve continuous yield improvement by characterising every risk and prioritising actions.


GAMP and GMP-Compliant Validation
GMP-compliant Validation. IVS systems and machines are designed according to GAMP & FDA validation. Allow IVS to take care of the validation of the automated inspection project, leaving your engineers to get on with their own busy schedules.
By using original IVS validation services, manufacturers ensure that their automated visual inspection systems and machines are tested and proven to the highest standard in a timely and cost-effective manner.
Device quality inspection systems and machines for automated visual inspection.
Interested in Device Quality Inspection Machines?
Get in touch today so we can answer any questions you have regarding our device quality inspection solutions. See how they can save you money, protect your brand and increase your yield.
Send us a message and we will respond as soon as possible.
Have a Question? Get in touch
Why you should use IVS automated visual inspection in your medical device and pharmaceuticals production.
Finds foreign matter in your production.
What’s the most common defect in drug production? Cellulose fibres – caused by towels, clothes, wipers and autoclave paper. This is followed by organic material and polyester fibres. For medical device production one of the most common defects in injection moulding are black inclusions, spots and bubbles. IVS automated visual inspection can detects these types of failures at high speed, allowing flaws and particulates to be identified and rejected before more value is added to the product.
Artifical Intelligence (AI) Deep Learning.
Our modern vision systems utilise the very latest AI and deep learning vision inspection technology. By combining traditional machine vision inspection methods, high resolution imaging cameras and deep learning – production quality can be improved by providing a mechanism for the automatic sorting of flawed and defective products. Even hard to spot cosmetic defects, particles, fragments and fibres can be eliminated, as well as shorts, flash and measurements for moulded medical devices such as syringe bodies, medical tubes, plungers, caps, inserts and closures.
To optimise your medical device production.
By utilising the latest generation machine vision for quality control of medical device and pharmaceutical production, users can benefit from the optimisation of production processes and production quality assurance. Gain real-time monitoring of all the issues on your lines by using innovate quality control vision inspection, innovate your factory and use the very latest vision system technology.
Automatically checks medical moulded parts.
If a failure in production is not picked up earlier it can sometimes lead to a whole hour, day, week or even a month’s worth of production being spoiled, or requiring inspection and impounding of an entire batch. Costly recalls, brand reputation and warranties can all be protected by using automated visual inspection on moulded components and assemblies in medical device manufacturing.