This month, we’re discussing the elements of semi-automatic inspection. This is the halfway house that pharmaceutical manufacturers have to move to from manual quality inspection (of individual products) at a bench when volumes start to increase but don’t warrant a fully automated inspection solution. The catalyst for change is when the inspection room is full of quality inspectors, and it’s time to increase the throughput of quality assessment. The semi-automatic inspection solution allows vials (liquid and lyo), syringes, cartridges and ampoules to be presented to the operator at speed to review before a magnifier, enabling manual rejection to be completed. This allows a single operator to do the job of a room of quality inspectors. This process mimics the standard GAMP manual inspection of the black and white background booths found in manual inspection, allowing for the throughput of high volumes. However, how does the semi-automatic inspection fit into the validation process and allow validation at the relevant levels?
![]()
Validation of such semi-automatic inspection must adhere to the United States Pharmacopeia (USP). The following chapters from the USP are regarded as the main literature and source for regulatory information surrounding the visual inspection of injectables:
Chapter 〈790〉 VISIBLE PARTICULATES IN INJECTIONS
Chapter 〈1790〉 VISUAL INSPECTION OF INJECTIONS
Chapter 〈788〉 PARTICULATE MATTER IN INJECTIONS
![]()
Chapter 〈790〉 establishes the expectation that each unit of injectable product will be inspected as part of the routine manufacturing process. This inspection should take place at a point when defects are most easily detected; for example, prior to labelling or insertion into a device or combination product. Semi¬automated inspection should only be performed by trained, qualified inspectors. The intent of this inspection is to detect and remove any observed defect. When in doubt, units should be removed
Defect Types
IVS semi-automatic inspection machines with operators allow for the inspection of cosmetic defects in the container and particulate matter within the fluid. The machines will enable the inspection of the following defects:
Cosmetic Defects:
• Glass cracks
• Scratches
• Missing stoppers/caps
• Improper cap/stopper closure
• Glass inclusions
![]()
Particulate Matter:
• Fibers
• Glass
• Metal
• Product Related
• Rubber
![]()
What are the particle definitions which apply to Semi-Automatic Inspection?
Extrinsic – Highest risk
Particles may originate from many sources. Those that are foreign to the manufacturing process are considered exogenous or “extrinsic” in origin; these include hair, non-process-related fibres, starch, minerals, insect parts, and similar inorganic and organic materials. Extrinsic material is generally a one-time occurrence and should result in the rejection of the affected container in which it is seen; however, elevated levels in the lot may implicate a broader contribution from the same source. These particles may carry an increased risk of microbiological or extractable contamination because less is known about their path before deposition in the product container or their interaction with the product.
Intrinsic – Medium Risk
Other particles are considered “intrinsic”, from within the process. Intrinsic particles may come from processing equipment or primary packaging materials that were either added during processing or not removed during container preparation. These primary product-contact materials may include stainless steel, seals, gaskets, packaging glass and elastomers, fluid transport tubing, and silicone lubricant. Such particles still pose the risk of a foreign body, but generally come from sterile or sanitized materials and more is known about their interactions when in contact with the product.
Inherent- Lower Risk
“Inherent” particles are considered the lowest risk as they are known to be or intended to be associated with specific product formulations. The physical form or nature of inherent particles varies from product to product and includes solutions, suspensions, emulsions, and other drug delivery systems that are designed as particle assemblies (agglomerates, aggregates). Product formulation-related particulate formation should be studied in the development phase and in samples placed on stability to determine the normal characteristics and time-based changes that can occur.
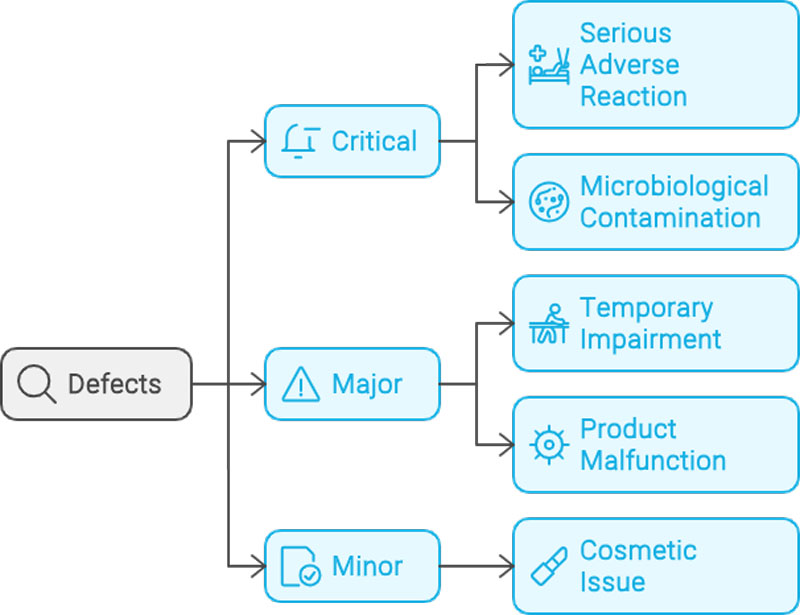
Defects are commonly grouped into classifications based on patient and compliance risk. The most common system uses three groups: critical, major, and minor. Critical defects are those that may cause serious adverse reaction or death of the patient if the product is used. This classification includes any nonconformity that compromises the integrity of the container and thereby risks microbiological contamination of the sterile product. Major defects carry the risk of a temporary impairment or medically reversible reaction, or involve a remote probability of a serious adverse reaction. This classification is also assigned to any defect which causes impairment to the use of the product. These may result in a malfunction that makes the product unusable. Minor defects do not impact product performance or compliance; they are often cosmetic in nature, affecting only product appearance or pharmaceutical elegance.
Inspection criteria
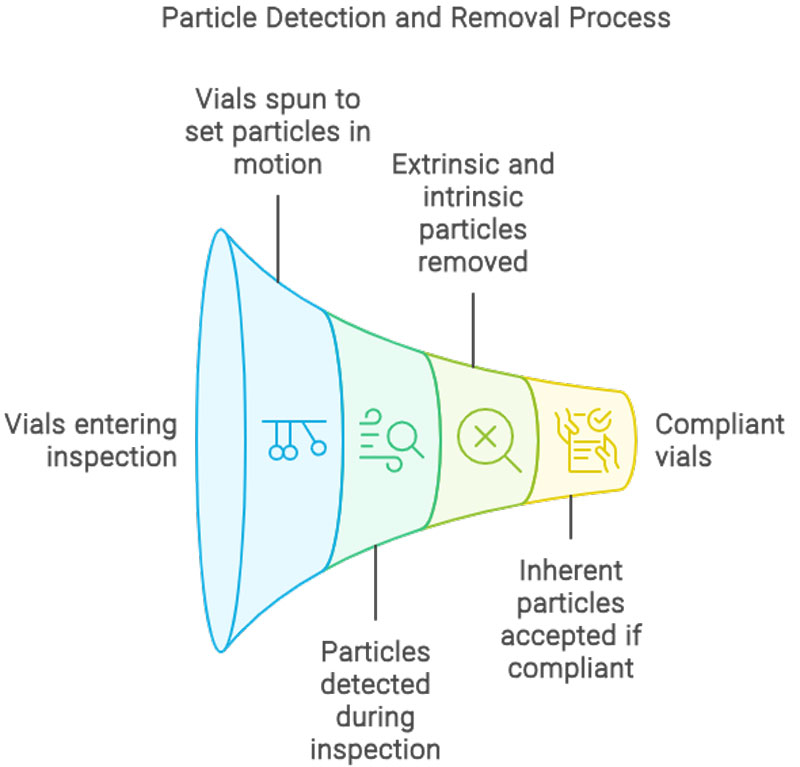
On semi-automatic inspection machines, the vials are spun up at speed prior to reaching the inspection area. This sets any visible particles in motion which aids in detection as stationary particles will be difficult to detect. Upon 100% inspection, visible extrinsic and intrinsic particles should be reliably removed. The test method allows inherent particles to be accepted if the product appearance specification allows inherent particle types. The size of particles reliably detected (2′::70% probability of detection) is generally 150 µm or larger. This Probability of Detection (POD) is dependent on the container characteristics (e.g., size, shape, transparency), inspection conditions (lighting and duration), formulation characteristics (colour and clarity), and particle characteristics (size, shape, colour, and density). For syringes the products are agitated and turned over so that no particulate matter is left in the bung and can be seen.
Critical Inspection Conditions
Light intensity
The results of the inspection process are influenced by the intensity of the light in the inspection zone. In general, increasing the intensity of the light that illuminates the container being inspected will improve inspection performance; 〈790〉 recommends light levels of 2,000-3,750 lux at the point of inspection for routine inspection of clear glass containers. Special attention should be given to assure that inspection is not performed below the lower limit of 2,000 lux.
Background and Contrast
Contrast between the defect of interest and the surrounding background is required for detection, and increased contrast improves detection. The use of both black and white backgrounds is described in 〈790〉, as well as other global pharmacopoeias. The use of both backgrounds provides good contrast for a wide range of particulate and container defects, which can be light or dark in appearance.
Inspection Rate
This is controlled by the roller travel speed. Sufficient time must be provided to allow for thorough inspection of each container; chapter 〈790〉 specifies a reference time of 10 s/container (5s each against both black and white backgrounds).
Magnification
Some inspection processes use a large magnifier to increase image size and thus increase the probability of detecting and rejecting containers with defects near the threshold of detection. Most lenses used during this inspection process are around X2 magnification.
Overall, Semi-Automatic Inspection is a necessary step when pharmaceutical manufacturers move into medium-volume production. Validating such systems allows production to ramp up to the required volume without the need for manual benches and provides capacity for future volumes before fully automated vision inspection with vision systems. More details on how to use Semi-Automatic inspection for regulatory compliance can be found here.
