What does every medical device manufacturer crave? Well, the delivery of the perfect part to their pharmaceutical customer, 100% of the time. One of the major stumbling blocks to that perfect score and getting a Parts Per Million (PPM) rate down to zero are the scratches, cracks and inclusions which can appear (almost randomly) in injection moulded parts. Most of the time, the body in question is a syringe, vial, pen delivery, pipette or injectable product which is transparent or opaque in nature, which means the crack, inclusion, or scratch is even more apparent to the end customer. Here we drill down on the top six defects you can expect to see in your moulded medical device and how vision systems are used to automatically find and reject the failure before it goes out of the factory door.
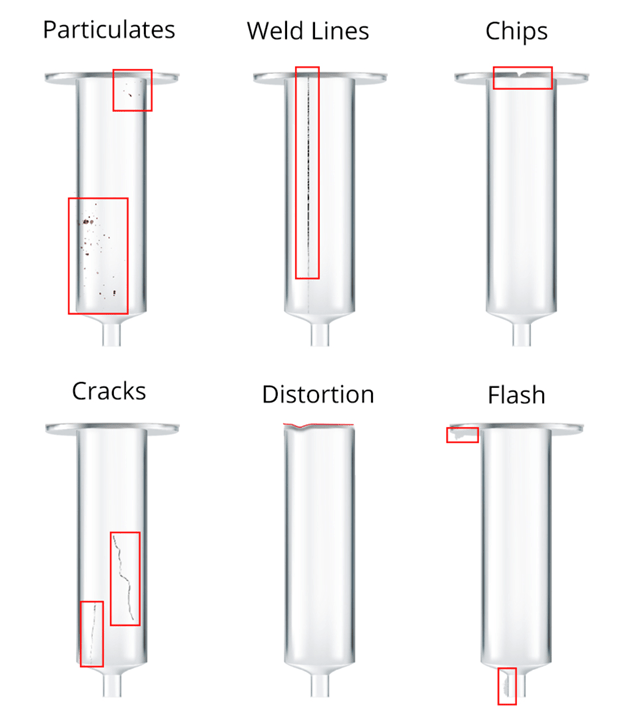
1. Shorts, flash and incomplete junctions.
Some of the failures are within the tube surface and are due to a moulding fault in production. For example, incomplete injections in mouldings create a noticeable short shot in the plastic ends of the product. The forming of junctions at the bottom end of the tube creates a deformation that must be analysed and rejected. Flash is characterised as additional material which creates an out of tolerance part.
2. Scratches and weld lines
These can be present on the main body, thread area or top shaft. They often have a slight depth to them and can be evident from some angles and less so from others. These could also be confused with weld lines which can run down the shaft or body of a syringe product, but this is also a defect that is not acceptable in production.
3. Cracks
Cracks in a medical device can have severe consequences to the patient and consumer; this could allow the pharmaceutical product to leak from the device, change the dosage or provide an unclean environment for the drug delivery device. Cracks could be in the main body or sometimes in the thread area, or around the sprue.
4. Bubbles, Black Spots and Foreign Materials
Bubbles and black spots are inclusions and foreign material, which are not acceptable on any product, but on a drug delivery device that is opaque or clear in nature, they stand out a lot! These sort of particulates are typically introduced through the moulding process and need to be automatically rejected. Often, the cosmetic nature for these sorts of inclusions is more the issue than the device’s functional ability based on the reject.
5. Sink marks, depressions and distortions
Sink marks, pits, and depressions can cause distortion in the components, thus putting the device out of tolerance or off-specification from the drawing. These again are caused by the moulding process and should be automatically inspected out from the batch.
6. Chips
Chips on the device have a similar appearance to short shots but could be caused on exit from the moulding or via physical damage through movement and storage. Chips on the body can cause out of tolerance parts and potential failure of the device in use.
But how does this automatic inspection work for all the above faults?
Well, a combination of optics, lighting and filters is the trick to identifying all the defects in medical plastic products. This combined with the ability to rotate the product (if it’s cylindrical in nature) or move it in front of many camera stations. Some of the critical lighting techniques used for the automated surface inspection of medical devices are:
Absorbing – the backlight is used to create contrasts of particulates, bubbles and cosmetic defects.
Reflecting – Illumination on-axis to the products creates reflections of fragments, oils and crystallisation.
Scattering – Low angle lighting highlights defects such as cracks and glass fragments.
Polarised – Polarised light highlights fibres and impurities.
These, combined with the use of telecentric optics (an optic with no magnification or perspective distortion), allows the product to be inspected to the extremities, i.e. all the way to the top and to the bottom. Thus, the whole medical device body can be examined in one shot.
100% automated inspection has come a long way in medical device and pharmaceutical manufacturing. Utilising a precision vision system for production is now a prerequisite for all medical device manufacturers.