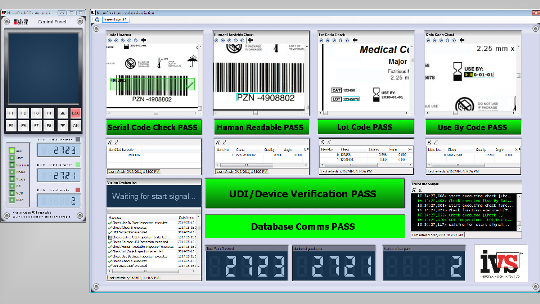
Device makers look to on-line vision systems to quality check the FDA’s unique device identifier requirements.
The Food and Drug Administration (FDA) in the US have released a final ruling requiring that most medical devices distributed in the United States must carry a unique device identifier (UDI). With the directive starting in September 2014 it has become increasingly important for device manufacturers to put the necessary quality control procedures in place now to confirm the UDI is both legible and traceable. As the FDA sees it, “A unique device identifier system has the potential to improve the quality of information in medical device adverse event reports, which will help the FDA identify product problems more quickly, better target recalls, and improve patient safety.” The UDI allows the device or product to be traced back to its roots in manufacturing.

This directive has implications for the whole of the medical device and pharmaceutical business but at a fundamental level during the production of the device or product the UDI needs to be applied and quality checked to confirm it meets the necessary specification.
Industrial Vision Systems (IVS) have been working with a number of companies to tackle these problems through the use of ever more sophisticated automated machine vision systems and processes. The FDA demands that automatic identification and data capture (AIDC) technology is used for the inspection process. By combining Optical Character Recognition/Optical Character Verification with print quality inspection and ID/code reading the UDI can be checked in real time through the whole production cycle to confirm compliance to the FDA specifications. The UDI can be applied to labels, boxes, products, components and devices – therefore a generic approach to the quality inspection needs to constructed. The UDI directive also states that all devices that will be implanted or sterilised must have permanent marks so the vision inspection system has to cope with confirming marks on varying material types, including implants.
Typically the code would be applied to include both a human readable and a machine readable mark, be it a 1D or 2D/Data Matrix code. Both need to be inspected to confirm they reach the necessary quality standards. For symbols this is according to the ISO standards 15415 and 15416. Digital cameras are used throughout the production process connected to a central IVS vision system which then communicates to the Factory Information System database to provide historical information and conformance. Full track and trace is now seen as the ideal solution. Most medical device companies run varied quality systems, data management and IT applications for their UDI requirements. The flexible user interface allows data storage, image storage and historical statistical process control (SPC) data to be easily managed independent of the background IT infrastructure.
These latest generation vision systems provide full audit logging and traceability to allow systems to be validated to Good Automated Manufacturing Practice (GAMP) standards. The UDI quality can be tracked through the process to make sure that it has not degraded through the production cycle and that multiple elements (e.g. inserts and instructions) have gone together correctly.
The UDI program will provide consumers with a better quality product, increased traceability against forgery and assurance that the product has been through the necessary production cycle and checks.
Further details can be found on the FDA website www.fda.gov/udi and information on quality checking the UDI can be found at www.industrialvision.co.uk/industries/medical-devices-pharmaceuticals