Good news from North America this week; total sales of machine vision systems and components saw 22% year-over-year growth in the first quarter of 2015, the highest first quarter total since the Automated Imaging Association (AIA) began recording statistics in the market. We are already seeing the ripples of this growth felt on UK shores over the last weeks and months.
In 2014, the North American vision market also experienced the best year since the AIA began recording statistics back in 2009. With 15.3% overall industry growth projected for 2015, representing a total of $2.2 billion, these most recent figures demonstrate that the industry is well on the way to living up to expectations and that software, such as vision technology, is likely to have the significant impact on the market in 2015. In fact, the machine vision systems arena saw a year-over-year increase of 24%.
Applications specific machine vision systems (ASMV) increased 24%. Additionally, machine vision components, including cameras, lighting, and software, saw an 11% growth in Q1 of 2015. Lighting grew by 28%, while cameras grew 11% and software 8%.
Overall, this is welcome and positive news for the UK manufacturing sector, it appears manufacturing companies are hiring more staff, with employment in the sector increasing for the 26th consecutive month.
At IVS, we continue to see interest and demand for machine vision products in industries such a medical device, pharmaceutical, and automation, which is great for the long term health of these markets.
With the North America machine vision market off to a solid start in 2015, it is clear to see that an increasing number of organisations and companies across various sectors are noting the value that machine vision and imaging systems can have on the bottom-line of their business.


 In terms of accessibility, the front of the enclosure cover can be easily removed to provide the user with full access to the vision system camera and optics, allowing changes to the focus and aperture settings.
In terms of accessibility, the front of the enclosure cover can be easily removed to provide the user with full access to the vision system camera and optics, allowing changes to the focus and aperture settings.



 Safecontractor is applicable to most sectors although it is particularly relevant to automotive, pharmaceutical and food manufacture, all of which are big users of contracted services. John Kinge, technical director of Safecontractor said, “Major organisations simply cannot afford to run the risk of employing contractors who are not able to prove that they have sound health and safety policies in place.”
Safecontractor is applicable to most sectors although it is particularly relevant to automotive, pharmaceutical and food manufacture, all of which are big users of contracted services. John Kinge, technical director of Safecontractor said, “Major organisations simply cannot afford to run the risk of employing contractors who are not able to prove that they have sound health and safety policies in place.”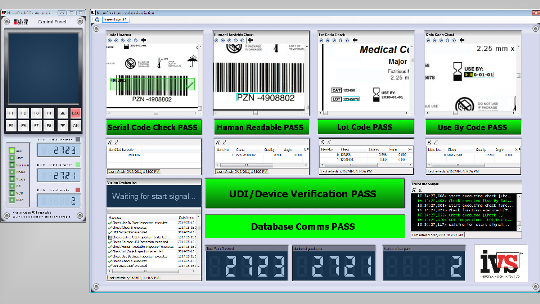

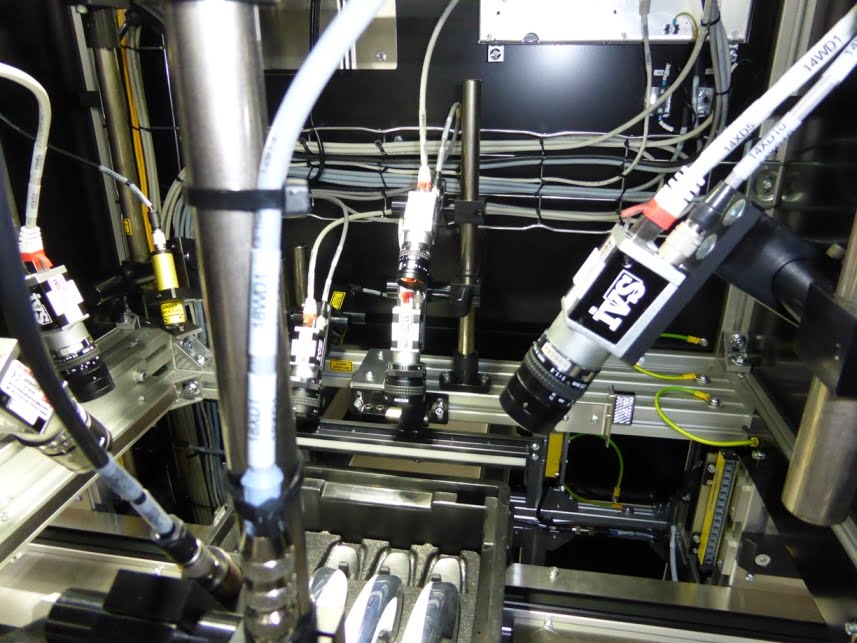

 IVS vision camera systems can be configured and deployed with ease thanks to plug and play support within the industry standard NeuroCheck® software suite. These latest generation machine vision cameras provide state-of-the-art digital technology, offering precise signal processing and superior image quality.
IVS vision camera systems can be configured and deployed with ease thanks to plug and play support within the industry standard NeuroCheck® software suite. These latest generation machine vision cameras provide state-of-the-art digital technology, offering precise signal processing and superior image quality.
